When you manufacture or handle medical devices, one worry never goes away: How do you sterilize sensitive products without damaging them?
Plastic syringes, IV sets, catheters, electronic sensors, and sealed medical packaging cannot handle high heat or moisture. Yet, they must be completely sterile to protect patients and meet strict regulations. This is where the ETO sterilization process becomes essential.
Ethylene Oxide (ETO) sterilization offers a safe, low-temperature way to kill bacteria, viruses, fungi, and spores without harming delicate materials. In this guide, we will explain how ETO sterilization works, step by step, in a simple and practical way.
What Is ETO Sterilization? (ETO Full Form & Meaning)
ETO stands for Ethylene Oxide. It is a colorless gas used to sterilize heat-sensitive and moisture-sensitive products.
Unlike steam or dry heat, ETO does not rely on high temperature. Instead, it uses controlled gas exposure to destroy microorganisms at low temperatures. This makes it ideal for products made from plastic, rubber, electronics, and complex packaging.
ETO sterilization is widely used in:
- Medical device manufacturing
- Pharmaceutical packaging
- Hospitals and CSSD departments
- Laboratory and research facilities
Why Ethylene Oxide Is Used for Sterilization
Many medical and pharmaceutical products cannot be sterilized using steam because heat and moisture can damage them. ETO is used because it is gentle on materials but strong against microbes.
ETO sterilization is commonly used for:
- Syringes and needles
- IV sets and tubing
- Catheters and surgical kits
- Blister packs and medical packaging
- Electronic medical devices
Industries that depend on ETO include healthcare, pharma, medical device manufacturing, and sterile packaging facilities.
Overview of the Ethylene Oxide Sterilization Process
The ethylene oxide sterilization process follows a controlled cycle from loading to aeration. Products are placed inside a sealed chamber where temperature, humidity, and gas levels are carefully controlled.
ETO gas penetrates packaging and tiny spaces inside devices. It damages the DNA of microorganisms, which prevents them from surviving or reproducing. This ensures complete sterilization even in hard-to-reach areas.
Temperature, humidity, and gas concentration are critical. If any of these are off, sterilization may be incomplete or unsafe.
ETO Sterilization Process Flow Chart
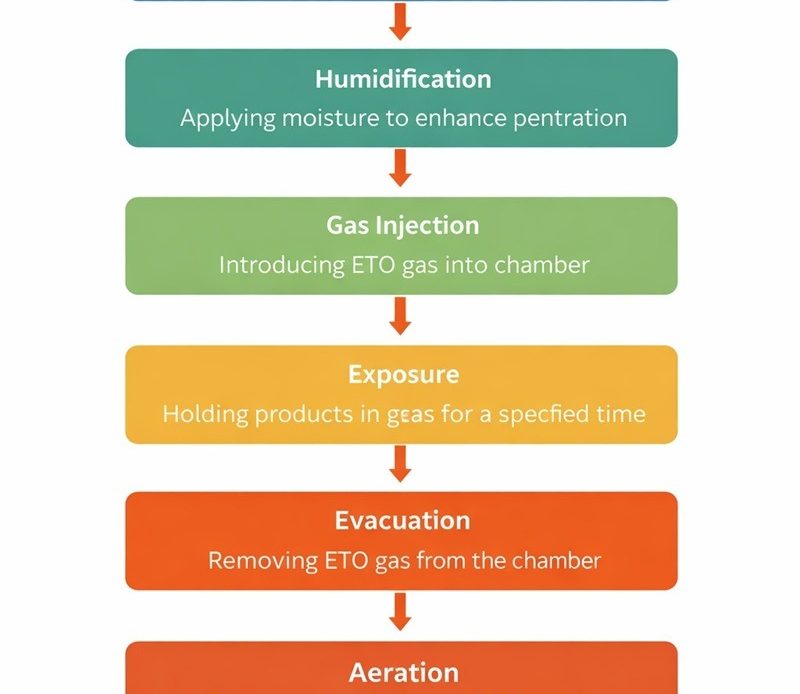
Here is the typical ETO sterilization flow:
- Pre-conditioning
- Humidification
- Gas Injection
- Exposure
- Evacuation
- Aeration
- Product Release
Each step ensures both sterilization and safety.
How Does the ETO Sterilization Process Work: Step-by-Step ETO Sterilization Cycle
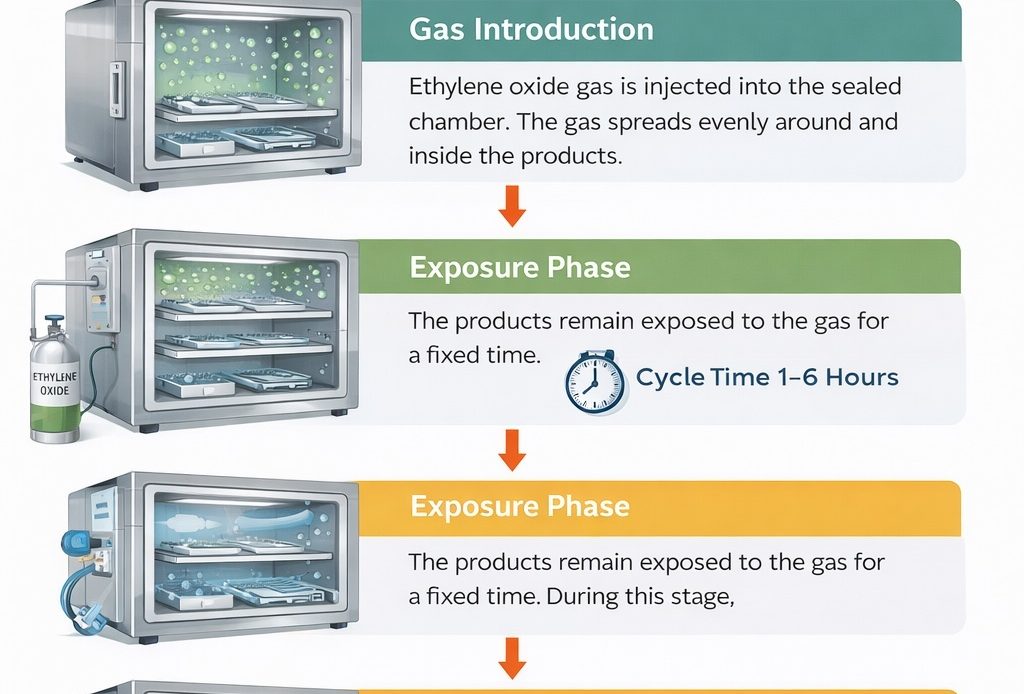
Pre-conditioning
Products are prepared in a controlled environment. Temperature and humidity are adjusted so the items are ready to absorb ETO gas.
Gas Introduction
Ethylene oxide gas is injected into the sealed chamber. The gas spreads evenly around and inside the products.
Exposure Phase
The products remain exposed to the gas for a fixed time. During this stage, ETO destroys bacteria, viruses, and spores.
Aeration & Degassing
After exposure, the gas is removed. Fresh air cycles through the chamber to remove all remaining ETO.
Final Validation
Biological indicators and sensors confirm that sterilization is complete and safe.
ETO Sterilization Temperature & Environmental Conditions
ETO sterilization is a low-temperature process, usually between 37°C and 60°C. This protects heat-sensitive devices.
Humidity helps ETO gas work better. Pressure controls allow the gas to reach deep into packaging and narrow spaces. Together, these factors ensure both safety and effectiveness.
How Long Does the ETO Sterilization Process Take?
A full ETO cycle usually takes 8 to 24 hours. This includes:
- Gas exposure
- Evacuation
- Aeration
Some products need longer aeration to remove all gas safely, especially dense or tightly packed loads.
What Is the Expiry Time After ETO Sterilization?
ETO sterilized products remain sterile as long as the packaging stays sealed and undamaged. Medical-grade packaging keeps the product sterile until it is opened. Proper storage in a clean, dry area is important to maintain shelf life.
Why ETO Is Trusted for Medical and Pharmaceutical Sterilization
Compared to steam, ETO is safer for sensitive products. It is approved by regulatory bodies because it offers deep sterilization without heat damage. Hospitals and manufacturers trust ETO because it meets strict ISO and safety standards.
How Equilateral Solutions Supports the ETO Sterilization Process
Equilateral Solutions designs ETO sterilization systems that offer precise control, high safety, and full compliance. Our machines support:
- Low-temperature gas cycles
- Leak detection and exhaust systems
- Validation and monitoring
- ISO and regulatory compliance
We also provide installation, training, and validation support.
FAQs About the ETO Sterilization Process
Is ETO safe?
Yes. When properly controlled and aerated, ETO is safe and widely used in healthcare.
Can all devices be sterilized with ETO?
ETO is ideal for heat-sensitive products. Metal tools that can handle heat may use steam instead.
How do you know sterilization is complete?
Sensors, biological indicators, and system monitoring confirm the cycle is successful.

